 資源預(yù)覽
資源預(yù)覽






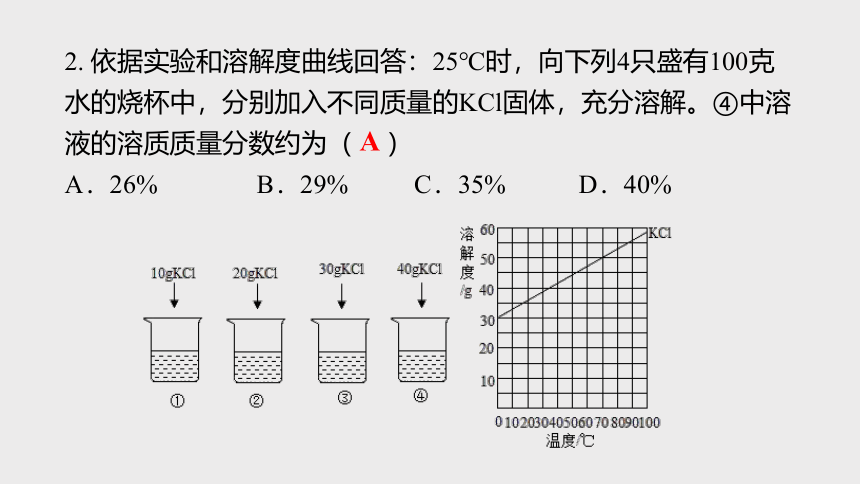
 資源預(yù)覽
資源預(yù)覽






